By Bishoy Morris Faltas, MD
Urothelial bladder cancer is the fifth most commonly diagnosed cancer in the United States resulting in 16,000 deaths annually. Until recently, progress in developing therapeutics for urothelial carcinoma has been slow. Platinum-based chemotherapy continues to be the first-line treatment for advanced urothelial carcinoma but ultimately the majority of patients will develop resistance. The recent development of immune-checkpoint inhibitors is promising but only one-fifth of patients respond to these agents. Currently, the majority of patients will ultimately die of chemotherapy-resistant or immunotherapy-resistant urothelial carcinoma. My goal is to develop a world-class bladder cancer research program to understand the fundamental biology underlying bladder cancer tumorigenesis and therapy resistance leading to major therapeutic advances. In the following comments, I want to frame what I see are the key elements needed to develop this research program at an already outstanding institution such as the Weill Cornell Medicine-Meyer Cancer Center. I have included examples from my research.
A) Therapeutic breakthroughs can only be built on a solid foundation of urothelial cancer biology
As a physician-scientist and a genitourinary medical oncologist, I recognize that the only way to accelerate the pace of therapeutic advances for urothelial carcinoma is to invest in basic studies of the biologic mechanisms that drive early tumorigenesis and resistance to therapy. My research aims to dissect these mechanisms through the lens of evolutionary biology. I adopt the view that tumorigenesis and treatment-resistance are phenotypes driven by certain molecular events that increase the “fitness” of urothelial cancer clones in the face of stochastic or deterministic selective pressures. My postdoctoral work has defined the clonal evolutionary dynamics of chemotherapy-resistant urothelial carcinoma (Faltas et. al Nature Genetics, 2016). I discovered that both divergent evolution and metastatic spread are early events in the natural history of urothelial carcinoma (Fig.1).
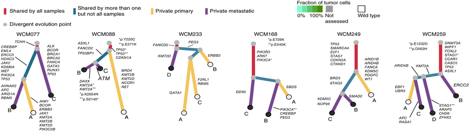
My study also revealed that APOBEC3-induced mutagenesis is clonally enriched in chemotherapy-treated urothelial carcinoma and continues to shape its evolution of throughout the tumors’ lifetime. (Fig. 2).
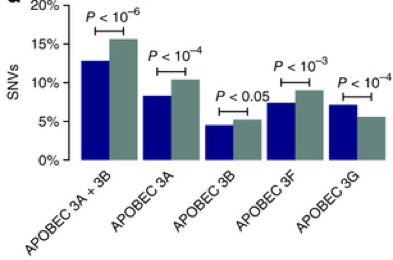
Figure 2. Enrichment of APOBEC3-indiced SNVs in chemotherapy-resistant urothelial carcinoma (Faltas et.al Nature Genetics, 2016).
These discoveries laid the foundation for my current research. My current work focuses on one fundamental biological question: what is the role of the APOBEC3 family of mutagenic proteins as evolutionary drivers of tumorigenesis and chemotherapy-resistance? I am using a multi-pronged approach combining genomics, functional cancer genetics (including CRISPR-Cas9 screens) and establishing new transgenic mouse models to answer this critical question. The results of these studies will identify the role of APOBEC3-induced mutagenesis in promoting genomic-instability and driving tumorigenesis and chemotherapy-resistance in urothelial carcinoma. My results will be critical the development of potential biomarkers for chemotherapy-response. They should aid in the discovery of targeted therapies to prevent or reverse platinum-resistance in UC. These findings will directly improve clinical outcomes in patients suffering from urothelial carcinoma. A three-year career development award from the Department of Defense currently supports this work.
B) Window-of-opportunity investigator-initiated trials: a new paradigm to translate laboratory insights
To lead in bladder cancer care internationally requires proposing and leading investigator-initiated trials. The Weill Cornell Medicine-Meyer Cancer Center genitourinary oncology program is already known for its strength in leading several trials for several genitourinary malignancies and immunotherapy. These trials must bubble up from basic and translational research observations conducted on our basic research program.
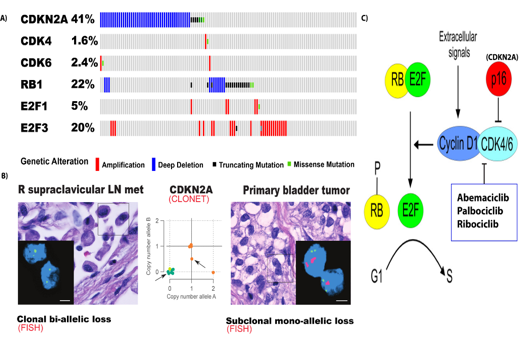
Figure 3. A) Frequency of molecular alterations in cell-cycle genes in MIBC (TCGA, 2014) B) Heterogeneity in CDKN2A copy-number within the same patient. Insets: CLONET bioinformatic allele-specific CDKN2A copy-number prediction for each tumor sample (middle), Fluorescent in situ hybridization validation: CDKN2A probe, green: reference probe. (Faltas et. al Nature Genetics, 2016) C) The CDKN2A-CDK4/6-RB pathway (adapted from (Peurala, Koivunen, Haapasaari, Bloigu, & Jukkola-Vuorinen, 2013).
The natural history and the current standard of care treatment for clinically-localized muscle-invasive bladder cancer (MIBC) offer a unique opportunity for window-of-opportunity clinical trials.
I have utilized this paradigm to translate basic observations I made in the laboratory into an investigator-initiated clinical trial.
My postdoctoral work previously demonstrated that MIBC is enriched with molecular alterations in cell-cycle genes (Faltas et. al Nature Genetics, 2016). CDKN2A copy-number loss is the most common alteration in cell-cycle genes and occurs in in 41 % of patients with MIBC (Fig.3A) (TCGA, 2014).We also identified a striking degree of molecular heterogeneity within a given MIBC patient (Faltas et al., 2016). This was made evident by the detection of multiples clones with discordant molecular alterations in the same patient. For example, I demonstrated the presence of several clones along a wide spectrum of CDKN2A copy-number status within an individual patient (Fig. 3 B). My study also revealed that heterozygous subclonal CDKN2A losses evolve into homozygous clonal deletions as the tumor progressed (Faltas et. al Nature Genetics, 2016)(Fig. 3B). These data provided the biological rationale for a window of opportunity clinical trial to define how CDK4/6 inhibition acts as a selective pressure shaping the genetic evolution of the disease (Fig.4). This will enable me to identify the molecular determinants of response to CDK4/6 inhibitors.
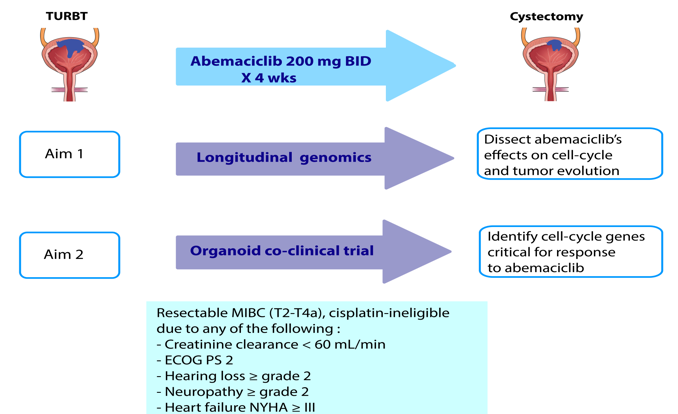
Figure 4. Trial schema: Following TURBT, abemaciclib will be administered for 4 weeks to cisplatin-ineligible MIBC patients enrolling in a window-of-opportunity clinical trial. Patients will then undergo radical cystectomy. Pre- and post- abemaciclib tumors will be collected.
Completion of this trial will have a positive impact by addressing the critical need for a targeted therapeutic strategy for cisplatin-unfit MIBC patients. The results will advance the field towards effective precision medicine and better clinical outcomes. I have obtained the pharmaceutical sponsor’s support for the trial. The sponsor will provide abemaciclib for enrolled subjects and additional funding for the trial and scientific correlatives through the Exploratory Investigator Sponsored Trials (ExIST) mechanism. My view is that this clinical trial paradigm is an ideal platform for testing novel therapeutic strategies and to gain a deeper biological understanding of tumor biology.
C) Personalized in vitro and in vivo cancer models to guide bladder cancer precision medicine.
Patient-derived models offer unique translational opportunities to advance therapy. Our group published the first report of patient-derived bladder cancer organoids (Fig. 5 (Pauli,…Faltas,…Rubin, Cancer Discovery, 2017).
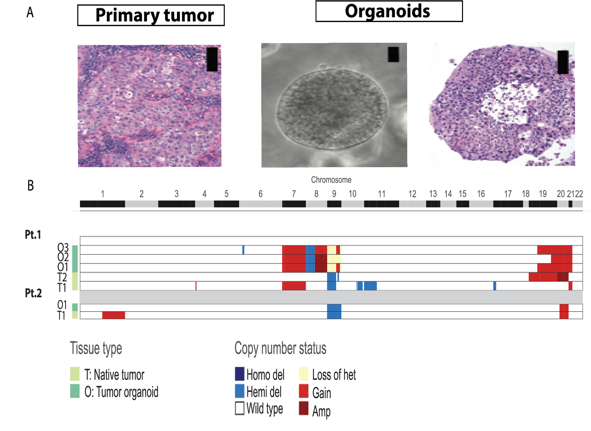
Figure 5. Representative patient-derived bladder cancer organoids are: A) morphologically and B) genetically concordant with patients’ primary tumors. Tumors and organoids show loss of heterozygosity (yellow) or deletion (blue) of 9p21 locus harboring CDKN2A. (Pauli,…Faltas,…Rubin, Cancer Discovery, 2017)
We created a robust precision cancer care platform that integrates whole-exome sequencing with a living biobank that enables high-throughput drug screens on patient-derived tumor organoids. This platform promotes the discovery of novel therapeutic approaches that can be assessed in clinical trials and provides personalized therapeutic options for individual patients where standard clinical options have been exhausted. For example, I am currently using primary patient-derived bladder cancer organoids and cell lines in a genome-wide CRISPR screen to identify molecular determinants of sensitivity to CDK4/6 inhibition (a collaboration with Francesca Dimechelis, University of Trento, Italy). Combining functional genetic screens with patient-derived organoids will allow us to advance our understanding of bladder cancer biology in a “real world” clinical context and to translate this understanding to precision medicine advances for bladder cancer patients. Establishing a bladder cancer organoid biobank at the Weill Cornell Medicine-Meyer Cancer Center will also serve the research community both locally and internationally in the question for development of novel drug targets and basic science discovery. This also represents possible partnerships with Pharma and biotech. Co-clinical trials using patient-derived organoids will be seamlessly integrated into early phase investigator-initiated clinical trials leading to a deeper understanding of predictors of sensitivity and resistance to new therapies.
D) A team-science approach to establish national and international scientific primacy in bladder cancer research
I believe that building research consortia and collaborations is the way forward to establish leadership in bladder cancer research at the national and international levels. This can be achieved by capitalizing on already existing research strengths at the Weill Cornell Medicine-Meyer Cancer Center and collaborating with other investigators at the national and the international level. I envision collaborations between the bladder cancer research program and my clinical colleagues in the genitourinary medical oncology program under the directorship of Dr. David Nanus and Dr. Scott Tagawa, I will also seek a strong partnership with the urology department especially with Dr. Douglas Scherr and Dr. Jim Hu. I will also capitalize on ongoing collaborations with Dr. Brian Robinson and Dr. Juan Miguel Mosquera in the department of pathology. The bladder cancer research program will also synergize with the outstanding scientific programs at the Weill Cornell Medicine-Meyer Cancer Center and an ongoing collaboration with Dr. Olivier Elemento at the Weill-Cornell Institute for Computational Biomedicine.
At the national level, I will continue to build on currently established collaborations and to form new ones. For example, my research has generated a neoantigenic map of advanced urothelial carcinoma (Faltas et al. ASCO GU oral presentation, 2016, JCO, 2016) and I have capitalized on this work to begin to establish several large collaborations. I am currently collaborating with several investigators to understand the role of neoantigens as predictors of response to immune-checkpoint blockade. I am currently part of the alliance correlative science team for several phase III clinical trials of immune-checkpoint inhibitors in urothelial cancer. These collaborations have resulted in grant applications to the NIH for funding through the Biomarker, Imaging and Quality of Life Studies Funding Program (BIQSFP) mechanism. I am also a member of a multi-institutional team of investigators from Weill-Cornell, UCSF, UNC and the University of Chicago who are involved in an effort to examine whether combined immune and neoantigenic signatures can be used to select patients that may benefit from different immunotherapy combinations. We have submitted a proposal for funding this study as a proposal for the Department of Defense Team Science Award. I anticipate that our studies will result in major insights into the contributions of neoantigens to the anti-tumor response during immune-checkpoint blockade. This will translate into positive outcomes for patients including new and effective biomarker and therapeutic strategies.
At the international level, I am participating in the International Bladder Cancer Network (IBCN). I have also started collaboration with a group of investigators in North Africa to understand the genomic landscape of schistosomiasis-induced squamous cell carcinoma of the bladder, which is endemic to this region.
E) Developing funds for research and programmatic growth through grants, philanthropy and crowd-sourcing
My goal is to establish full research independence by obtaining several R01s within the next five years. In the long term, the success of the bladder cancer research program will ultimately lead to programmatic grants. To diversify funding sources and to meet the challenge of restricted NIH funding will require active fundraising. I plan to be very involved in fundraising efforts and to work with all stakeholders in bladder cancer research including patients, foundations and the public in fund raising and to help expand the philanthropy base using various fundraising activities such as crowd sourcing. Engaging potential and current donors through committees and special interest discussions will require close collaboration with the Weill Cornell Medicine-Meyer Cancer Center development specialists to draw attention to bladder cancer research. This will enable the bladder cancer research program to expand into additional scientific and clinical niches and to recruit and retain the necessary talent for making breakthroughs.


